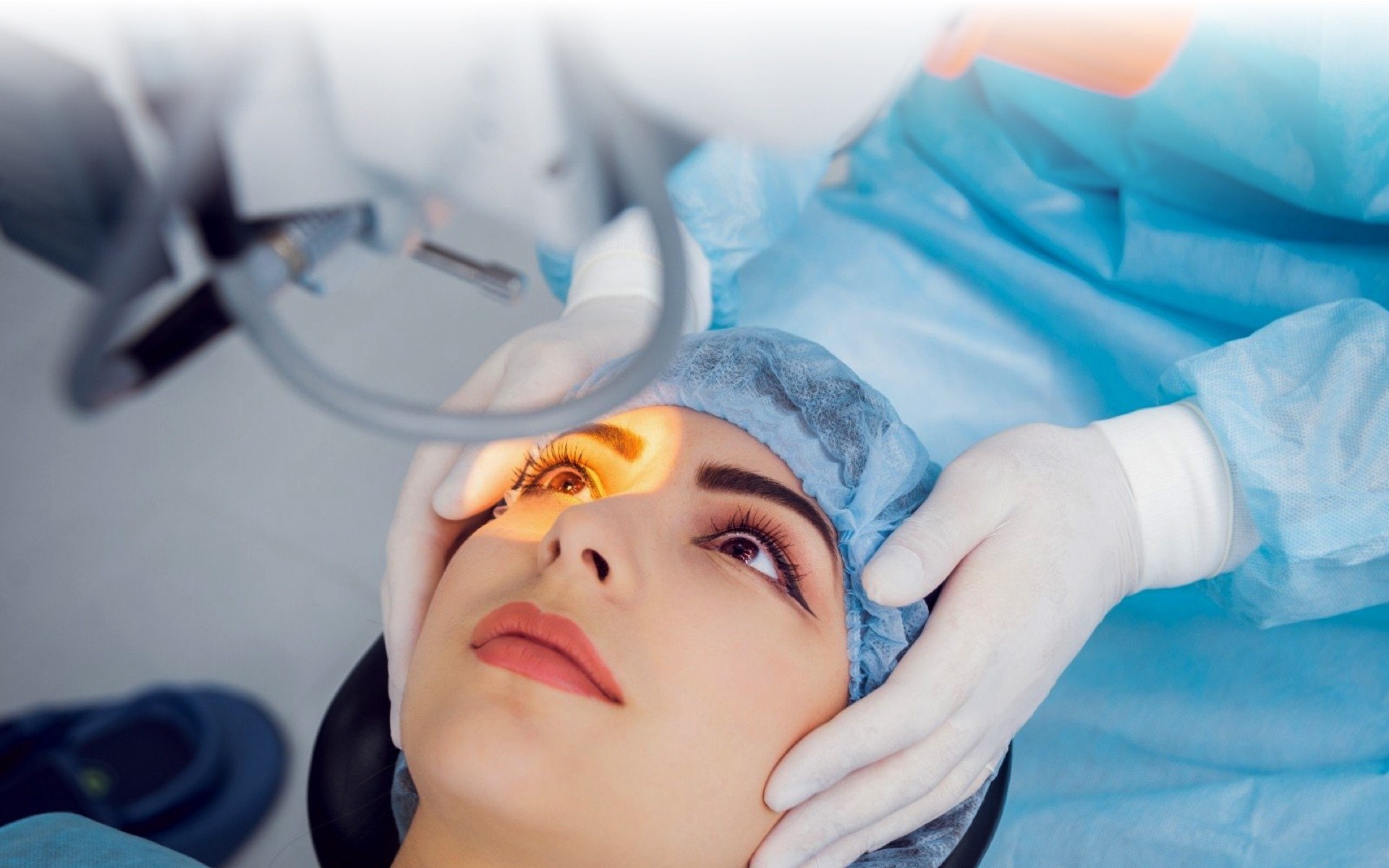
iFix Medical Pty Ltd is an Australian medical device company commercialising innovative technology and products for ophthalmologists to better manage and treat corneal disease, corneal repair and corneal transplantation. iFix Medical has research and device development associations with the Save Sight Institute at University of Sydney and TRICEP at University of Wollongong.
iFix Medical has developed a novel medical treatment technology for corneal diseases and injuries.
PRODUCTS
CORNEA REPAIR PRODUCT
Next generation corneal treatment and repair.
3D Bioprinting and innovative technology and products for corneal disease and repair of damage to the cornea.
iFix Medical has developed a novel medical treatment technology for corneal diseases and injuries. The iFix System comprises two components: iFixInk™ and the iFixPen®. The iFixPen is a hand-held device that delivers a 3D-printed structure directly onto corneal defects. The patent pending iFixInk creates a completely transparent and biodegradable structure to seal the wound, prevent pathogen infiltration, relieve pain and accelerate healing.



DRY EYE PRODUCT
Dry eye effects up to 50% of the population.
Research and development of minimally invasive treatments with superior efficacy for dry eye.
iFix Medical has initiated a research and development program to assess the efficacy of several novel medical treatments for dry eye disease. The six candidates, IFX-005 to IFX-010, utilise the company’s proprietary iFixInk platform composition and target different biological pathways with different device functional features. Exciting times at iFix Medical.
Corneal & Dry Eye Disease
The cornea is the clear window at the front of eye and corneal disease is the third most common cause of blindness worldwide. Corneal ulceration is extremely painful and accounts for 55,000 presentations to hospital each year across Australia. Current medical treatments do not adequately address the issues of pain relief, infection or the development of scar tissue. Infection and scarring may necessitate lengthy hospital stays and further treatment.
Dry eye disease affects people of all ages and symptoms range from intermittent irritation to significant pain, visual loss, discomfort and reduction in quality of life. Published literature incidence rates vary from 5–50% of the population. Currently, there is no known cure, only treatment of symptoms. Treatment options reflect the severity of the condition and, due to the chronic nature of the disease, may require ongoing and often frequent application of medication, eye drops or other therapies. The optimal treatment for dry eye disease would be a minimally invasive product that allows for simple compliance to provide prolonged symptom reduction.
TEAM

Experienced MedTech CEO and has established, managed and raised capital for private and ASX listed startups, developing and commercialising novel medical devices for international markets.

Corneal specialist and ophthalmic surgeon with 20+ years’ experience and professional connections with The University of Sydney; NSW Organ & Tissue Donation Service, the Corneal Unit at Sydney Eye Hospital and the Australian Ocular Biobank.

Experienced researcher and project manager with a proven track record of delivering novel and innovating ophthalmic research.
Supporters & Partners




CONTACT
Get in touch with us for more information on news, product development and investor opportunities.